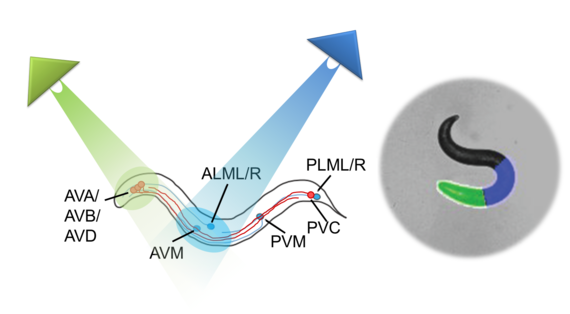
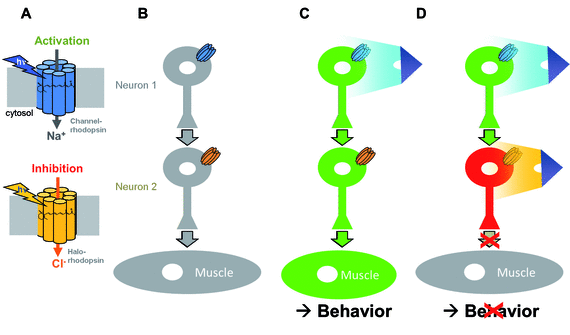
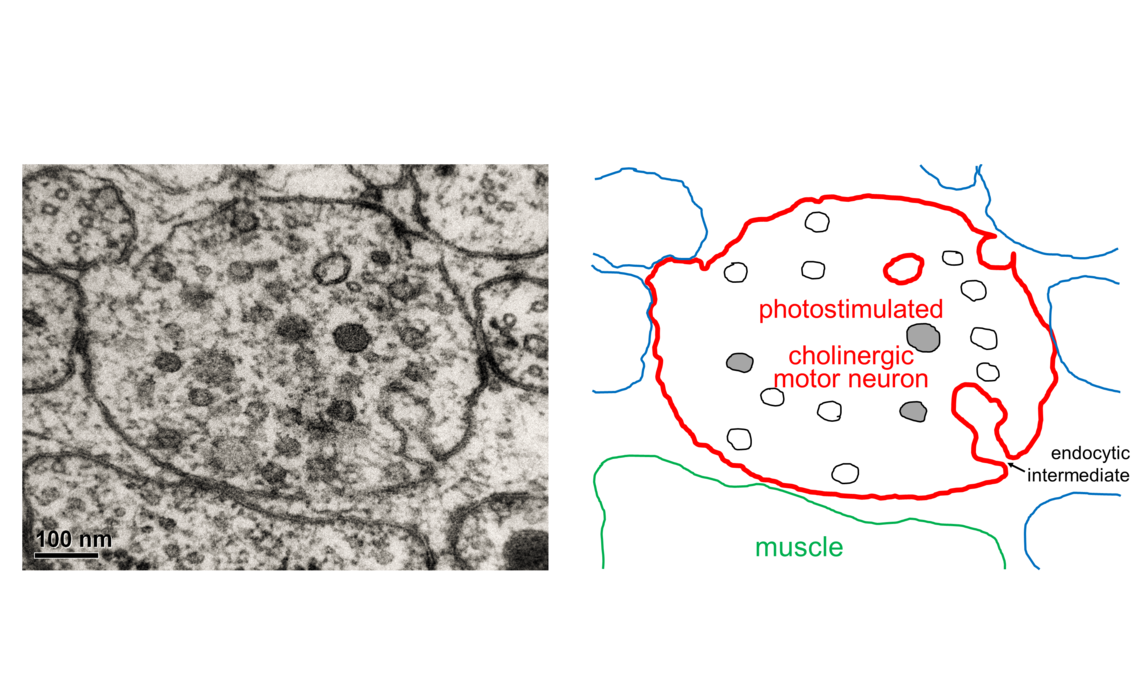
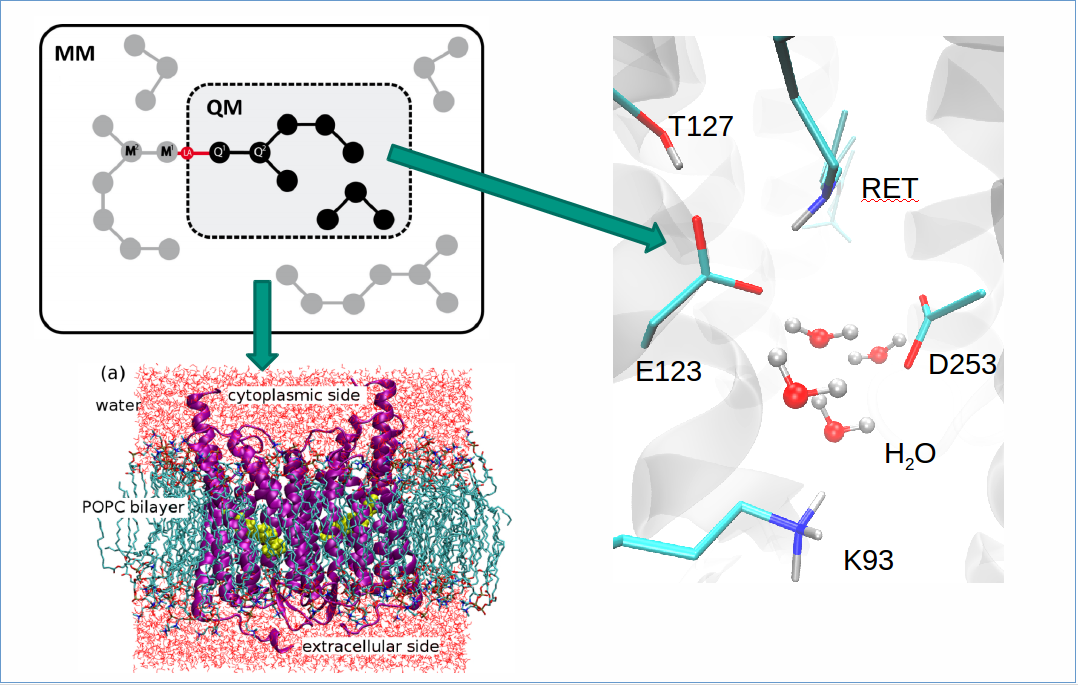
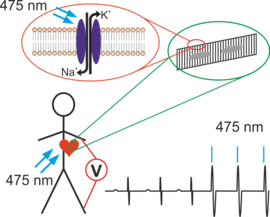
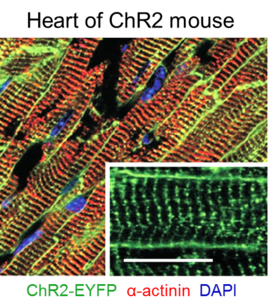
What is Optogenetics?
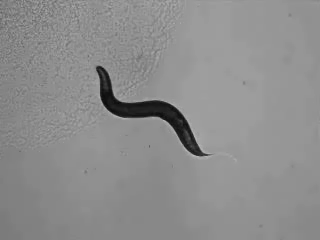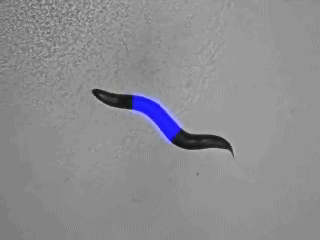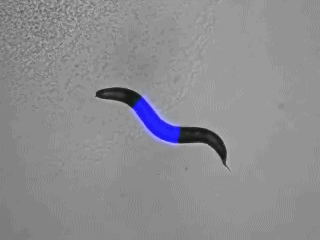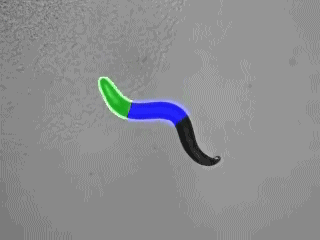
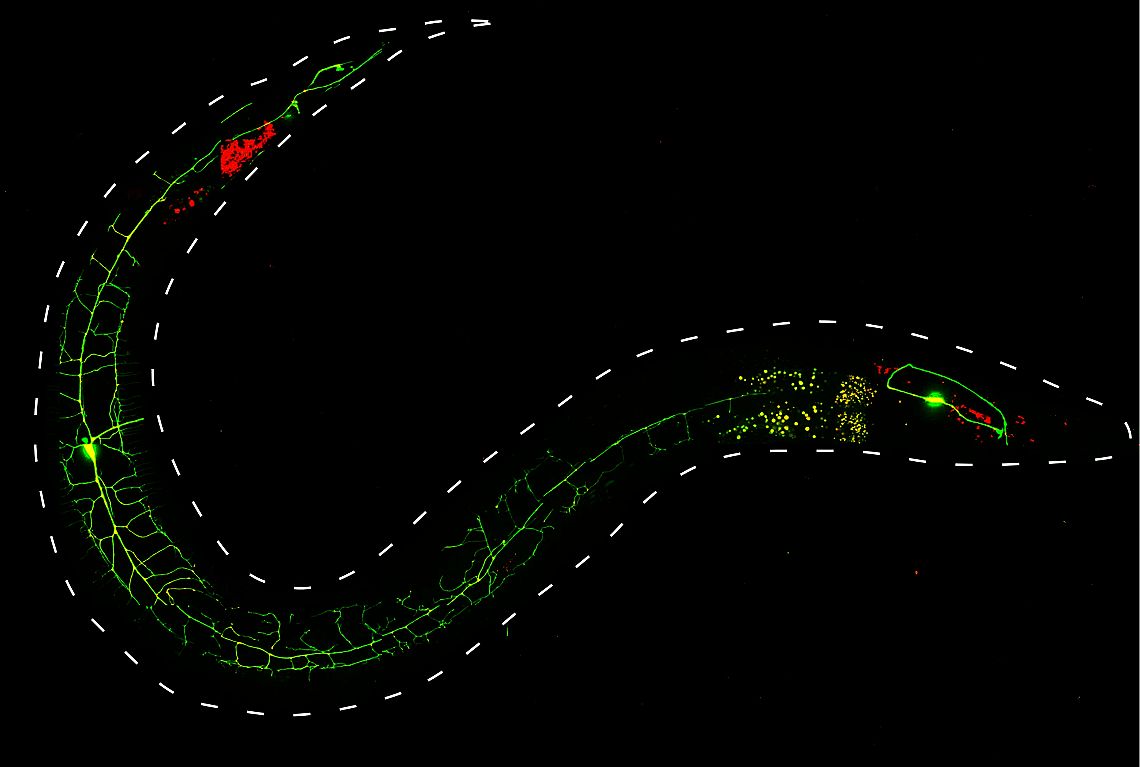
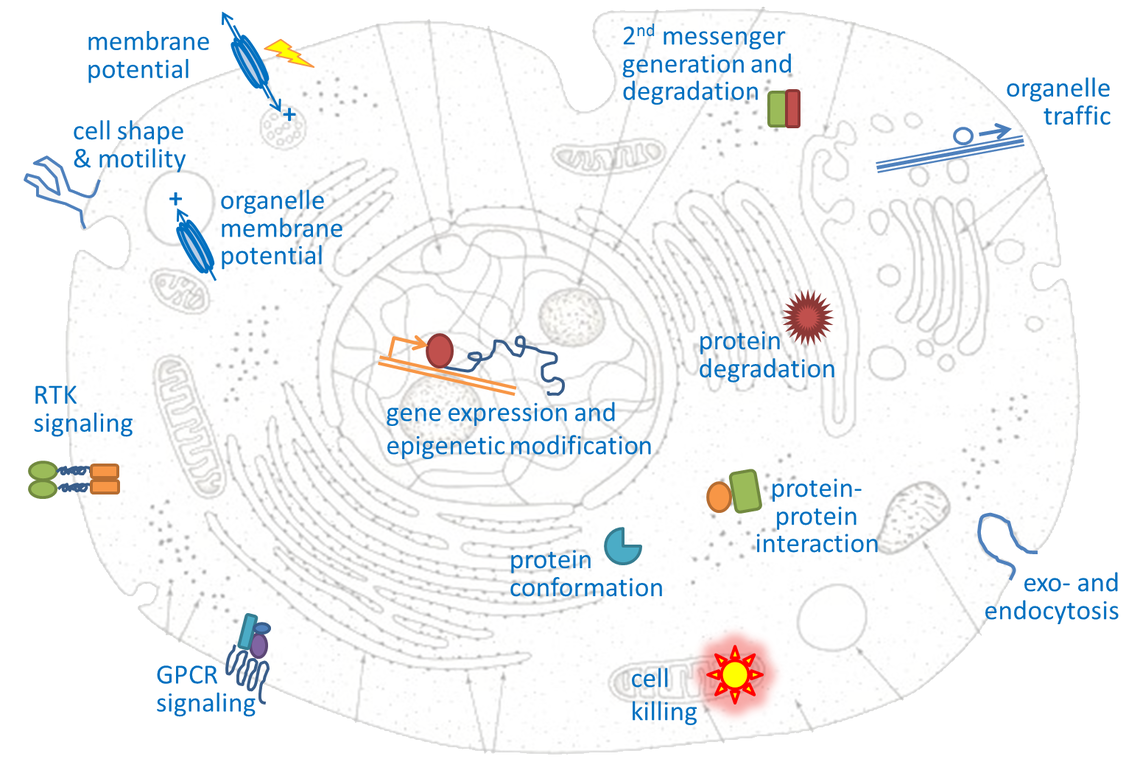
“Optogenetics” summarizes methodologies and applications that utilize genetically encoded (or addressable) light-controlled proteins or molecules, which enable temporally precise control of molecular, cellular or (neuronal) network activities in live cells, tissue and animals. Optogenetic actuators are proteins found in natural light-sensitive organisms (often, these are microbes), whose genetic code is transfered to cells of higher animals, for example laboratory 'model' animals like nematodes, flies, fish and mice. These actuators, for example the famous channelrhodopsins, enable manipulating ion fluxes and membrane voltage. Upon illumination, these proteins open an ion-selective pore, thus leading to altered membrane voltage within milliseconds, and thereby, in the case of neurons or (cardiac) muscle cells, activating the cell. The consequence is precisely triggered neuronal firing, or muscle twitching, for example leading to locomotion of the animal or a heart beat. An optogenetic 'counterplayer' of channelrhodopsin is halorhodopsin, that pumps chloride ions into cells, thus inhibiting their activity. Other optogenetic actuators generate so-called 2nd messengers, which modify cellular signaling or the biochemistry of the cell. Further optogenetic tools were developed that allow the researcher to control gene expression, protein interaction or degradation, or cell death. Apart from natural or engineered light-sensitive proteins, optogenetic tools can be proteins that are made photoswitchable by small chemical compounds, which are often linked physically to the protein of interest. Last, optogenetics also encompasses genetically encoded optical sensors for Ca2+, membrane voltage, pH, small molecules, or 2nd messengers.